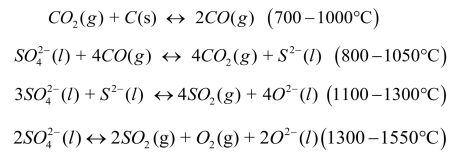Mathematical simulation of glass melting processes |
||
|
|
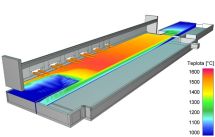
Mathematical modeling is traditional tool for the analysis of glass melting processes. CFD methods (Computed Fluid Dynamics) simulation based on the transfer of mass, momentum and energy calculate velocity and temperature of the melt in the melter. Figure shows the calculated temperature fields on the melter top melt surface. |
|
Bubble behaviour in glass melts |
||
|
|
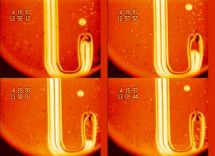
Gaseous or solid inclusions are the most common cause unacceptable product defects and may adversely affect the operation of the device. For a description of their behavior in the melt, we use a combination of experimental and mathematical techniques. The experiments reveal the mechanism of controlling phenomena, provide the input data and verify the mathematical model. Mathematical description, which is based on a set of differential equations describing the rate of change of the bubble size and composition is usually applied to the pre-computed velocity and temperature fields. Figure shows the sequence of visual observation of changes in bubble size, which is used to verify the mathematical description of the interaction of bubbles with the melt. |
|
Nucleation of bubbles |
||
 |
Methods of high-temperature observation and image analysis were used to determine the temperature at which bubbles nucleate at a platinum wire immersed in the glass melt. Bubbles growing during slow linear increase in temperature were identified and their diameter measured. The dependence between the diameter of bubbles and the temperature was extrapolated to zero size of the respective bubbles, and thus determines the temperature of nucleation. |
|
Chemical reactions evolving gases |
||
 |
In particular, reactions of sulfur compounds with reducing or oxidizing agents affect nucleation and separation bubbles, foaming of the melt and dissolution of the refractory particles.
Influencing the reactions kinetics can optimize the melting process. |
|
Corrosion of refractories by melts |
||
 |
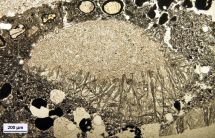
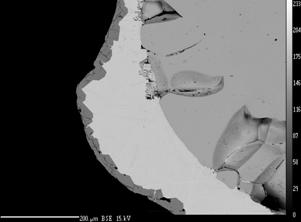
Isothermal static or dynamic corrosion tests follow the mechanism and kinetics of corrosion processes, evaluates changes in the microstructure of the refractories and predict the amount resulting from defects in the glass - blisters and crystalline inclusions. Figure on the left shows an arrangement of the plate corrosion test. Figure on the right illustrates the change in microstructure of refractory materials due to corrosion by an alkali melt - the conversion of mullite grain to secondary tabular corundum. |
|
Electrochemical processes at the interface of electrode and molten glass |
||
|
|
The causes and mechanisms of corrosion, development of bubbles and the release of condensed reaction products at the interface of materials, in particular molybdenum, platinum and tin-oxide based material are studied. The following experimental techniques are used: the electrical potential monitoring, the evaluation of the direct electrode mass losses, the study of the interface electrode-melt by SEM analysis (Figure on the left - SEM image of the excluded Sb under a layer Mo2S3 on the Mo electrode) and visual observation of high-temperature processes in melt (Figure on the right - Development of oxygen bubbles on a Pt electrodes in a borosilicate glass melt under the passage of electrical current). |
|
Identification of bubble sources in industrial melting space |
||
|
|
For the expected sources the laboratory measurements of the bubble size distribution, composition and release frequency of bubbles into the melt is performed. The results create a knowledge data base. Experimental values forms also the boundary conditions of the mathematical model of the bubble behavior in the melting furnace. The comparison of product defects analyses and the values obtained from the database and the results of the model makes possible to locate the source of the defects. Figures show examples of experimental tracking the bubble sources – determination of the size distribution and the number of bubbles released from the refractory at the bottom and walls of the melter (left) or from the interface between the batch and the melt (right). |
|
Selected papers |
||
|
||
Glass melting processes and their modelling
Updated: 14.11.2015 20:19, Author: Jaroslav Kloužek